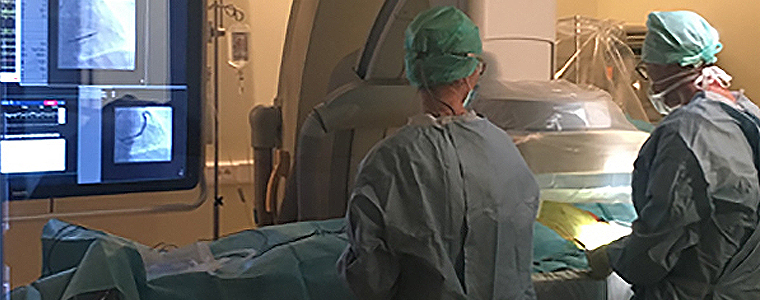
The first implantation of Axone as part of the ASTRAL-4LV study was successfully performed on December 3, 2020, by Professor Frédéric Anselme at the Rouen University Hospital in France. The purpose of the ASTRAL-4LV study is to assess the chronic safety and performance of the Axone lead for cardiac resynchronization therapy (CRT).
“Approximately 30% of patients do not respond to CRT therapy, and one of the main reasons is the difficulty of being able to pace the left ventricle in the correct locations.” said Professor Frédéric Anselme. “Axone makes it possible to find left ventricular pacing sites that are inaccessible with standard leads. Axone is what we needed to improve the response rate to CRT and have a positive impact on patient outcomes”.
ASTRAL-4LV is a prospective, single arm, multicenter clinical trial that will enroll 152 patients in 20 centers in France, Germany, Italy, Netherlands, Portugal, Spain, and Austria.
For more details, click here. An article has been published in French here.